Eligibility and Exclusion Criteria for Participants
Inclusion Criteria: patients who are
- Patients with newly diagnosed depressive disorder (> 9 on the Physical Health Questionnaire Version 9 [PHQ-9] screening at baseline in the last two years
- Patients who are on 1 or 2 anti-depressants prior to participation in the trial
- Aged between 18 years and 65 years
- Have provided written informed consent
- Have completed sixth grade or higher education in English language to respond to standard questionnaires
Exclusion Criteria: patients are excluded if they have
- Patients on more than 2 anti-depressants at time of recruitment
- Acute myocardial infarction in the past 12 months
- Suffering from congestive heart failure
- Are pregnant and lactating
- History of epilepsy or seizures
- Uncontrolled hypertension diagnosed by a physician (consistently elevated systolic blood pressure ≥ 150mmHg or diastolic blood pressure ≥ 90mmHg)
- History of concussion in the past 12 months
- History of stroke
- Uncontrolled diabetes (HbA1c > 6.5%)
- History of chronic pain
- History of autoimmune condition
- History of Bipolar Disorder
- History of Autism Spectrum Disorder (ASD)
- History of Attention Deficit Hyperactivity Disorder (ADHD)
- History of neurodevelopmental disorder (e.g., Intellectual disability)
- History of neurodegenerative disorders (Alzheimer’s disease, Parkinson’s disease, Huntington’s disease etc.)
- History of Schizophrenia Spectrum Disorder
- History of malignant tumor
- History of liver failure
- History of renal insufficiency
- History of substance misuse treatment
- History of or planned bariatric surgery within 6 months of pharmacogenomic testing
- Participants not willing sign the informed consent
- Participants unable to respond to standard questionnaires in English
Study Procedures
Physician’s Referral
If a patient meets the above criteria and is interested to participate in the trial, please refer the patient/ participant to PPI by:
- Email to PPI at research@personalizedprescribing.com with patient’s name, email, telephone number OR
- Ask patients to contact PPI directly by telephone at 1-844-943-0210 ext. 263 or email at research@personalizedprescribing.com. In case the patient contacts us directly, they are required to provide the referral physician’s information to proceed.
Participant Screening for Eligibility and Consent
Once the referral is received by PPI, a pharmacist will contact the participants by telephone to:
- Screen the patient/ participant for eligibility
- If eligible, the pharmacist would discuss the consents and request the participant to sign the consents (digitally); participants must consent that their physician receives a copy of their report
- If participants agree to proceed, they need to answer pharmacist’s intake questionnaires (related to diagnosed condition, lifestyle, medication history etc.)
- Intake questionnaires also include questionnaires to calculate depression scale scores and medication related side-effects, if any.
- The telephone call may take as much as 30 minutes.
Sample Collection and Testing
When participants complete the screening and consent process, PPI will send a saliva sample kits to participant’s address within 2-5 days of the screening. The participants are encouraged to send the sample back at their earliest convenience, ideally within 1-2 days of receiving the kit.
Personalized Prescribing Inc. analyzes the samples in their own laboratory, accredited by College of American Pathologist (CAP). Patients’ report will be completed within 10 business days after receiving the sample in PPI laboratory.
At this time, participants that returned their samples will be paid the first $50.00.
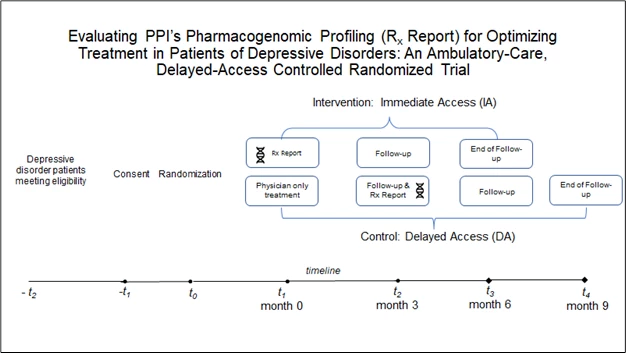
Randomization and Recommendation
As soon as the report is ready, the Principal Investigator/ Study Coordinator will use a computerized program to randomize the participants in Immediate-Access (Treatment) and Delayed-Access (Control) groups.
For Immediate Access (Treatment) group:
- The participants will be sent two emails: one containing the password protected link to the report Rx report, and the other containing a one-time password for accessing the report. Once the participant accesses the report the first time, they will be promoted to change the password.
- The pharmacist will contact the participant in the next 7 days (5 business days) by phone to discuss the report. Their physicians will receive the report by fax. Physicians are not obligated to follow our pharmacist’s recommendations.
- Follow-ups will be conducted 90 days after sending the report to the participant by email. If participants do not reply to the follow-up email, a Research Assistant will call participants to follow-up and discuss if the participants have reviewed the report with their physician and whether their medications were changed.
- Participants will receive the remaining $50 after the primary follow-up.
- The secondary outcome questionnaires will be completed via email after 6 months (lifestyle and customer satisfaction questionnaires).
For the Delayed Access (Control) group:
- The participants will be sent an email advising them that they are in the control group and will not receive their Rx Report at this time.
- The participants will be suggested that they should inform their physician to proceed with treatment as usual (TAU).
- 90 days after the TAU, an email will be sent to the participants to follow-up and obtain responses of primary outcome questionnaires based on their physician-initiated medication changes without the Rx Report.
- At that time, the Delayed Access participants will receive the Rx Report recommendation and will have access to PPI’s pharmacist services. Rx Report will be sent by two emails, with login link and password in separate emails and participants will be advised to change the one-time password as soon as possible.
- The pharmacists will explain the Rx Report to participants and fax the report to their physicians.
- Participants will receive the remaining $50 after the primary follow-up.
- If participants and their physicians decide to use Rx Report recommendation, we will follow-up again in 3 and 6 months to obtain participant’s primary and secondary response to measure effectiveness of Rx Report and services.
Follow-up Expectation
Based on the randomization date or the date the report is sent to participants, all participants are expected to cooperate with PPI by responding to our emails and answering questions (medication changes, side effects and outcomes of the medication used etc.) asked by the research assistant and pharmacists, as required. The assessments during follow-up will include the questionnaires like PHQ-9, GAD-7, MADRS, Q-LES-Q-SF, and CSQ.
Day-to-day management
A dedicated research coordinator will be involved with the pharmacists in the screening and obtaining consent from the research participants. While the study is open label, we will have a research assistant who is not aware of the group allocation will conduct and record the outcome data (questionnaires) under the supervision of the coordinator.